Etiometry Announces CE Mark and Health Canada Authorization for Adult Use of AI-Based Algorithm that Detects Risk of Hypercapnia
News and Press Releases
Previously authorized for pediatric populations, critical care teams in Europe and Canada can now utilize Etiometry’s IVCO2 IndexTM to determine the likelihood of inadequate carbon dioxide ventilation in adults
BOSTON, Mass. – March 21, 2023 – Etiometry, the leader in clinical decision-support software for critical care, today announced CE Mark and Health Canada authorization of its IVCO2 Index™ for adult populations, which allows clinicians to visualize inadequate ventilation of carbon dioxide with other contextual data from the Etiometry Platform to help inform intervention decisions and get ahead of patient deterioration. The IVCO2 Index was first FDA-cleared for pediatric use in 2019 and now carries CE Mark and Health Canada licenses for both pediatric and adult use.
On the heels of January’s FDA clearance of its IDO2 Index for adults – which was previously licensed for adult use by Health Canada and CE Marked in 2022 – Etiometry continues to seek authorizations for all of its risk algorithms to be used for both pediatrics and adults in the U.S., Canada and Europe.
“We are serious in our pursuit to expand authorizations of all four of our current risk indices,” said Shane Cooke, CEO of Etiometry. “What drives us forward is knowing how our precision analytics bring situational awareness to strained care teams to help mitigate risk and enhance patient outcomes.”
Using mathematical models of human physiology to determine the likelihood a patient is experiencing inadequate carbon dioxide (CO2) ventilation or hypercapnia, Etiometry’s IVCO2 Index continuously tracks the probability that a patient’s arterial blood gas sample has a partial pressure of CO2 (PaCO2) greater than 50 mmHg – with the ability to select patient-specific thresholds at the bedside.
The IVCO2 Index provides care teams an additional safety net to detect deterioration in patients with conditions that require tight control of PaCO2 or when V/Q mismatch can hamper the management of PaCO2.
The IVCO2 Index, along with Etiometry’s other risk indices, indicate the probability a patient will experience a harmful physiologic state and can also be embedded into the Etiometry Platform’s growing list of automated clinical pathways to improve length of stay and decrease ventilation time.
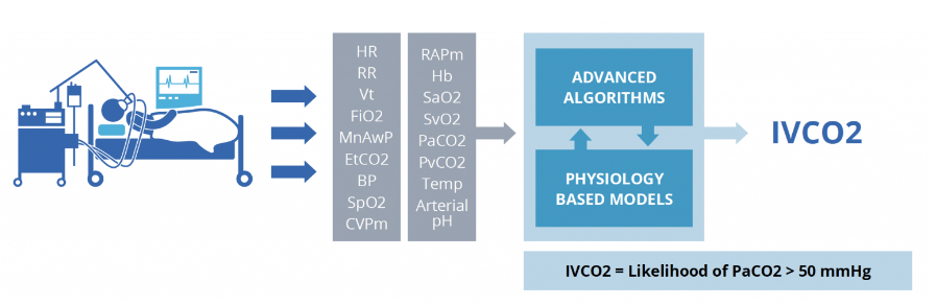
Etiometry’s IVCO2 IndexTM – derived using key vital signs and laboratory results collected by the Etiometry Platform – determines likelihood of inadequate carbon dioxide ventilation.
###
About Etiometry
Founded in 2010, Etiometry is the leader in clinical decision-support software designed to help clinicians in the intensive care setting make data-based decisions regarding their patients’ care and treatment. The company’s technologies provide valuable clinical insight and analysis to support early recognition of subtle changes in patients’ conditions to avoid complications and speed recovery. Etiometry has eight FDA clearances and four Health Canada approvals and CE markings. With roots in pediatric ICUs, Etiometry’s software is utilized by some of the world’s top academic medical centers, 20 of the Best Children’s Hospitals, ranked by U.S. News & World Report, 4 out of 5 of the highest ranked children’s hospitals in Newsweek’s Top Specialty Hospitals in the World and a growing number of top adult hospitals. Etiometry is committed to improving patient outcomes, increasing clinical efficiency, and lowering the cost of care through the more effective use of data.
The Etiometry Platform is an end-to-end data management software solution for the collection, analysis, visualization, and archiving of ICU clinical data. It is designed to facilitate the use of all available data to support the anticipation and management of the dynamic condition of patients requiring intensive care. To learn more, visit www.etiometry.com.
Media Contact:
Andrew Young
P: 615-603-1574
E: andrew@hencove.com


