Digital Health Company Earns FDA Clearance for Second Patient Risk Index
News and Press Releases
BOSTON, December 10th, 2019 – Etiometry Inc., a leading digital health company, today announced the FDA clearance of their second algorithm, the Inadequate Ventilation of Carbon Dioxide (IVCO2) Index™. The IVCO2 Index, along with the already cleared Inadequate Delivery of Oxygen (IDO2) Index™, will be displayed on Etiometry’s critical care software platform which is utilized in top hospitals throughout the world.
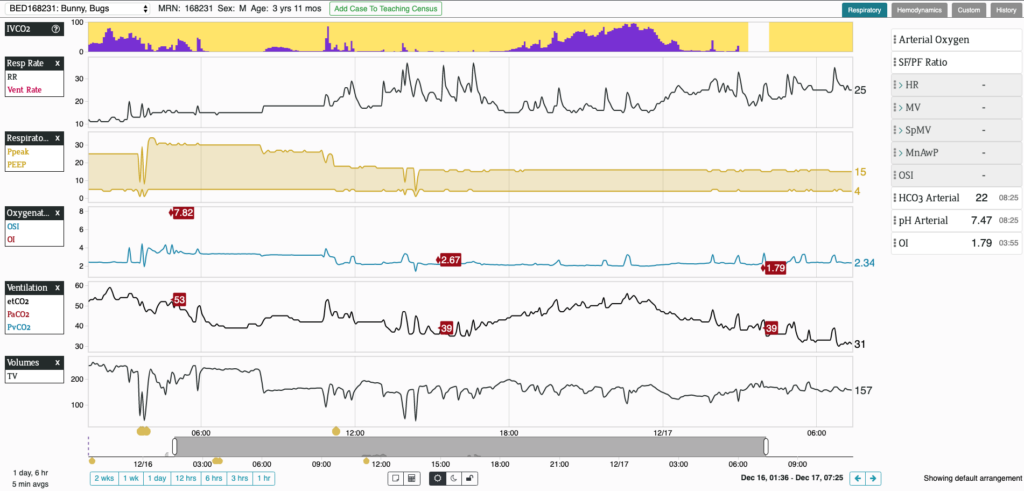
“The IVCO2 Index has the capability to merge multiple data sources to continuously track ventilation-perfusion mismatch and detect inadequate ventilation in mechanically ventilated patients,” said Dimitar Baronov, co-founder and CTO, “it has been specifically designed to supplement and enhance existing conventional monitoring such as end-tidal CO2 and bring attention to patients who need it most.”
Etiometry is the world’s first company to earn FDA clearance for two critical care algorithms. Today’s announcement marks a new chapter in the company’s growing portfolio of dynamic patient-specific analytics, which are scalable across multiple patient risks.
Etiometry’s technology platform includes T3 Data Aggregation & Visualization Software which enables clinicians to see important data for their patients in near real-time, including key vital signs and parameters, lab results, medical device inputs, history and trends, and dynamic patient-specific algorithms (IDO2 and IVCO2) to help inform clinical decisions. Additionally, the company’s Quality Improvement System enables hospitals to review collected data, generate quality reports to inform their practice, and conduct retrospective studies on patients and the impact of different treatment paths.
“We are focused on advancing data science, and the release of IVCO2 highlights the strength of our team, and the scalability of our platform” said Shane Cooke, President & CEO of Etiometry. “We are committed to improving patient care and will continue to bring technology advancements to the market which empower clinicians to make the best decisions for their patients.”
About Etiometry
Etiometry Inc. is the leader in clinical decision-support software for the intensive care environment. Our technologies provide valuable clinical insight and analysis to support early recognition of subtle changes in patient condition to avoid complications and speed recovery. Etiometry is committed to improving patient outcomes, increasing clinical efficiency, and lowering costs of care through the more effective use of all available data.
For more information, visit www.etiometry.com.